
Surgically Targeted Radiation Therapy (STaRT) for patients with operable brain tumors
Right Treatment. Right Time. Right Place.
GammaTile Therapy is a Surgically Targeted Radiation Therapy (STaRT) that provides immediate, dose-intense treatment at the completion of resection. By getting a head STaRT on fighting the tumor, resection plus GammaTile Therapy can extend local recurrence-free survival with minimal complications, reduced patient burden, and assured compliance.[1]
Elevating Outcomes With Optimized Dosimetry
GammaTile Therapy vs Intensity-Modulated Radiation Therapy (IMRT)
GammaTile is designed with uniform radiation-source spacing to ensure an even dose distribution. The favorable depth-dose profile optimizes local tumor control.[1-5]
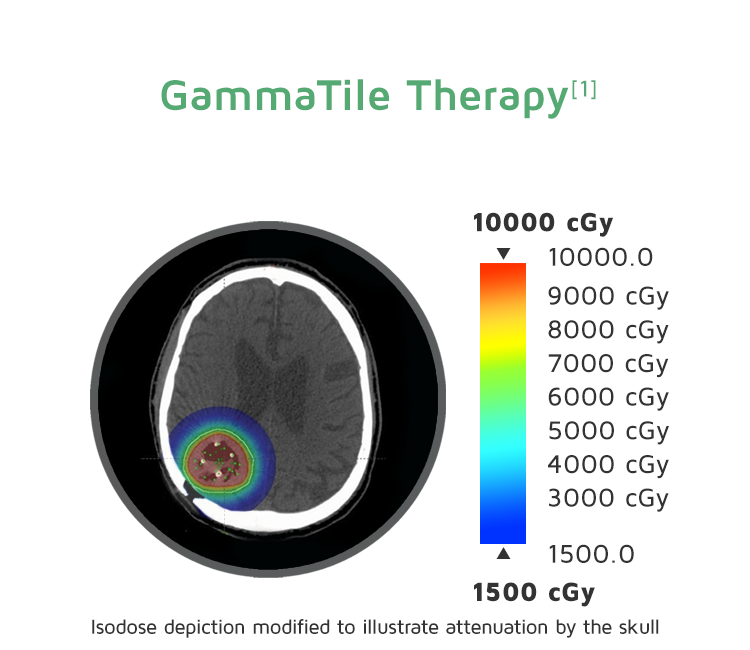
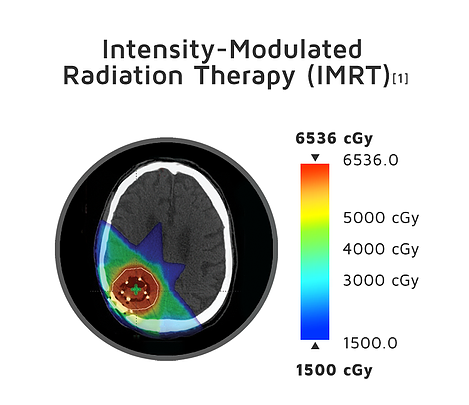
The colors indicate the radiation location and intensity from the 2 types of treatment. Blue-green indicates lower radiation levels. Red indicates higher levels. The extracranial dose is likely lower than shown, as the planning system did not utilize inhomogeneity corrections.
Proven Efficacy
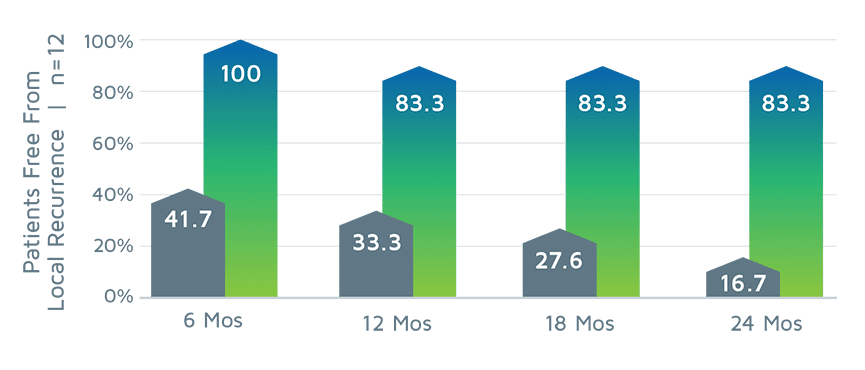
LOCAL CONTROL: RECURRENT BRAIN METASTASES AND RECURRENT MENINGIOMAS
GammaTile Therapy significantly improves local control vs the previous standard-of-care treatment in patients with recurrent brain metastases and recurrent meningiomas.[2,4]
RECURRENT BRAIN METASTASES[4]
RECURRENT MENINGIOMAS[8]
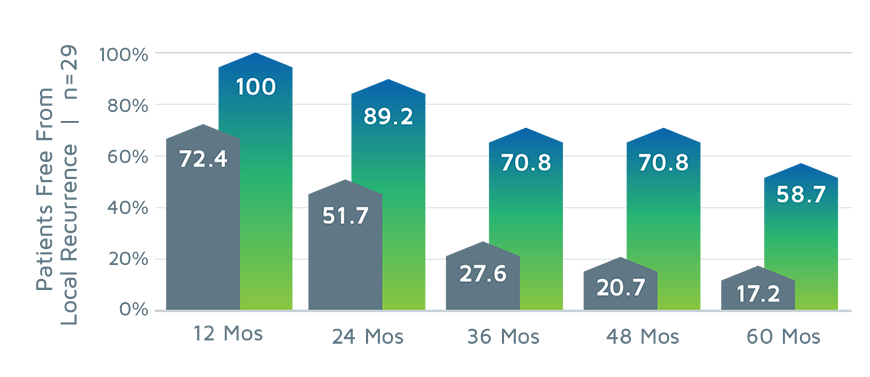

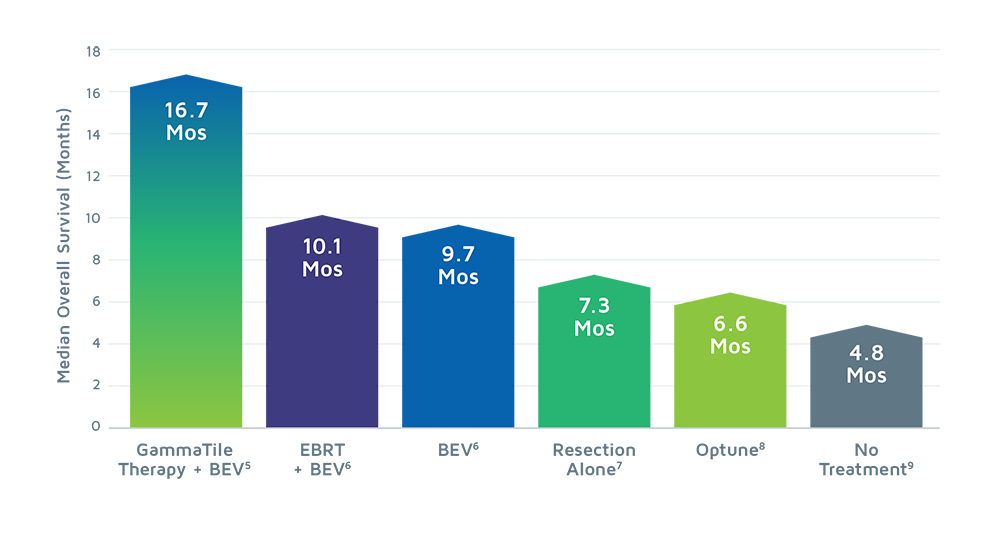
OVERALL SURVIVAL: RECURRENT GLIOBLASTOMA [5]
GammaTile Therapy demonstrates a potential for improved overall survival when comparing the effectiveness of surgery plus GammaTile Therapy to other treatment modalities across different clinical studies in patients with recurrent glioblastoma (GBM).
Advancing Safety
Designed with the brain’s delicate environment in mind to limit radiation changes in healthy tissue[1]
RADIATION-RELATED ADVERSE EVENTS

How It Works. An Eloquent Solution.
GammaTile Therapy targets tumor cells while preserving brain tissue. Surgically guided treatment of the local radiation dose to the operative bed optimizes the therapeutic margin while minimizing complications.[1]
Structural offset of the radiation source from the brain tissue prevents harmful direct seed-to-tissue contact and enables intraoperative adjustment.
Achieve local control with optimized dosimetry
- 50% of the therapeutic dose is delivered within the first 10 days after surgery, which helps prevent residual tumor cells from replicating.[2]
- 88% of the therapeutic dose is delivered within 30 days, with more than 95% of the dose delivered by 6 weeks.[2]
- A favorable depth-dose profile optimizes local tumor control.[2-5]
Cesium-131 Distribution and Intensity

Bioresorbable, conformable collagen tile preserves healthy tissue
• GammaTile uses a Cesium-131 radiation source, which has a half-life of 9.7 days[2-5]
• Favorable depth-dose profile optimizes local tumor control[2-5,9]
• Uniform radiation-source spacing ensures a predictable, therapeutic radiation dose
• Its design helps limit the radiation exposure to medical staff and caregivers[6]
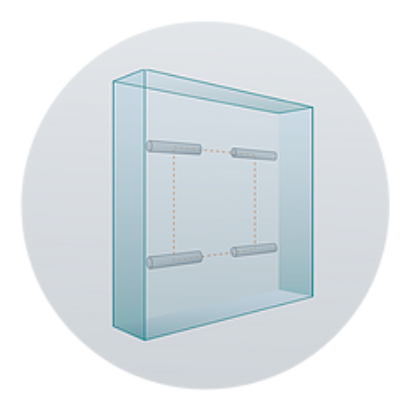
Uniform Radiation Source,
Structurally Offset Design
2-cm height | 2-cm width | 4-mm thickness

Learn more about GammaTile Therapy
Visit our patient site to explore patient testimonials, webinars, publications, and more
REFERENCES
- Brachman D, et al. Surgically Targeted Radiation Therapy: Safety Profile of Collagen Tile Brachytherapy in 79 Recurrent, Previously Irradiated Intracranial Neoplasms on a Prospective Clinical Trial. Brachytherapy 18, S35–S36 (2019).
- Brachman D, et al. Resection and permanent intracranial brachytherapy using modular, biocompatible cesium-131 implants: results in 20 recurrent, previously irradiated meningiomas. J Neurosurg 131, 1819–1828 (2019).
- Nakaji P, et al. Resection and Surgically Targeted Radiation Therapy for the Treatment of Larger Recurrent or Newly Diagnosed Brain Metastasis: Results From a Prospective Trial. Cureus 12, e11570 (2020).
- Imber B et al. Salvage resection plus cesium-131 brachytherapy durably controls post-SRS recurrent brain metastases. J Neuro-oncol 159, 609–618 (2022).
- Gessler D, et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neuro-oncology Adv 4, vdab185 (2021).
- Smith K, et al. Safety and patterns of survivorship in recurrent GBM following resection and surgically targeted radiation therapy: Results from a prospective trial. Neuro-oncology 24, S4–S15 (2022).
- Tsien C, et al. Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): initial outcomes and RT plan quality report. International Journal of Radiation Oncology, Biology, Physics. 2019;105(1):578.
- Kutuk, T. et al. Surgically targeted radia1on therapy (STaRT) for recurrent brain metastases: Ini1al clinical experience. Brachytherapy (2023) doi:10.1016/j.brachy.2023.08.002.
- Dharnipragada, R. et al. GammaTile® (GT) as a brachytherapy planorm for rapidly growing brain metastasis. Neuro-Oncol. Adv. 5, (2023).
GammaTile® is indicated as a treatment for patients with newly diagnosed malignant intracranial neoplasms and patients with recurrent intracranial neoplasms. For full product and safety information, refer to the Instructions for Use.
